Trailer: Calling Bullsh!t is Back
We’re back. Join us as we ask weight loss app Noom to step on the scale, analyze Robinhood’s aim to “democratize finance for all” and consult with some real experts […]
 play_arrow
play_arrow
Whistleblowers: A very special kind of bs detector Calling Bullsh!t
 play_arrow
play_arrow
McKinsey: Something to hide? Calling Bullsh!t
 play_arrow
play_arrow
Web3: A more humane, egalitarian, and decentralized internet? Calling Bullsh!t

 play_arrow
play_arrow
Juul Update: A Hazy Future? Calling Bullsh!t

Reporter at Bloomberg News & Author of “The Devil’s Playbook: Big
Tobacco, JUUL, and the Addiction of a New Generation”
In this bonus episode, we pick up where we left off with Juul since our Season 1 episode in February. We ended that episode with Juul in regulatory limbo, awaiting FDA authorization and still selling e-cigarettes. And on July 23, 2022 – after a long two-year review – Juul got their decision. In what was considered an unusual decision to many, the FDA ordered Juul to stop selling its products immediately.
But, the surprises with the FDA’s decision just keep coming. So, in this BS update episode, we’re joined by an old friend to discuss what this means for Juul and just what the heck is going on at the FDA.
This was a company that wanted to attain unicorn status that eventually did. And there were a lot of investors that made a lot of money.
Had they chosen to go a more plodding kind of responsible path, they might have ended up making Juul, but Juul might not have ever seen the light of day because no investor in Silicon Valley would’ve invested in [that] company.
-Lauren Etter
MUSIC: “In Passage” by Migration
CNBC
[SOT] The FDA is preparing to order Juul to pull its e-cigarettes off the market as early as this week…
TY MONTAGUE (VO)
That’s a CNBC interview from the morning of June 23rd, 2022 between Squawk Box host Joe Kernen and former FDA Commissioner Scott Gottlieb.
CNBC
[SOT] I suspect the FDA is going to look beyond the application and look at the history of this product and that’s going to be a factor in how they weigh their decision. So if they do reject Juul – assuming it’s not on some technical issue related to the application itself then I would – my hunch would be that it would turn on the legacy associated with this particular product.
TY MONTAGUE (VO)
Juul has been selling its products here unregulated since 2015. It took five years for the FDA to even request an application and another two years for them to review Juul’s submission.
On the afternoon of June 23rd – just hours after the Kernen – Gottlieb interview, the FDA finally announced that decision.
CNBC
[SOT] After a two-year review the US Food and Drug Administration announced today that it will ban all vaping and e-cigarette products sold by Juul.
TY MONTAGUE (VO)
Some cheered. Others booed. But most were just scratching their heads by the ensuing carousel of perplexing plot twists.
And since Juul was a Season 1 fan favorite, we wanted to see how this new news affects Juul’s BS score.
If you haven’t listened to the original episode, go check it out. We’ll be waiting for you when you get back.
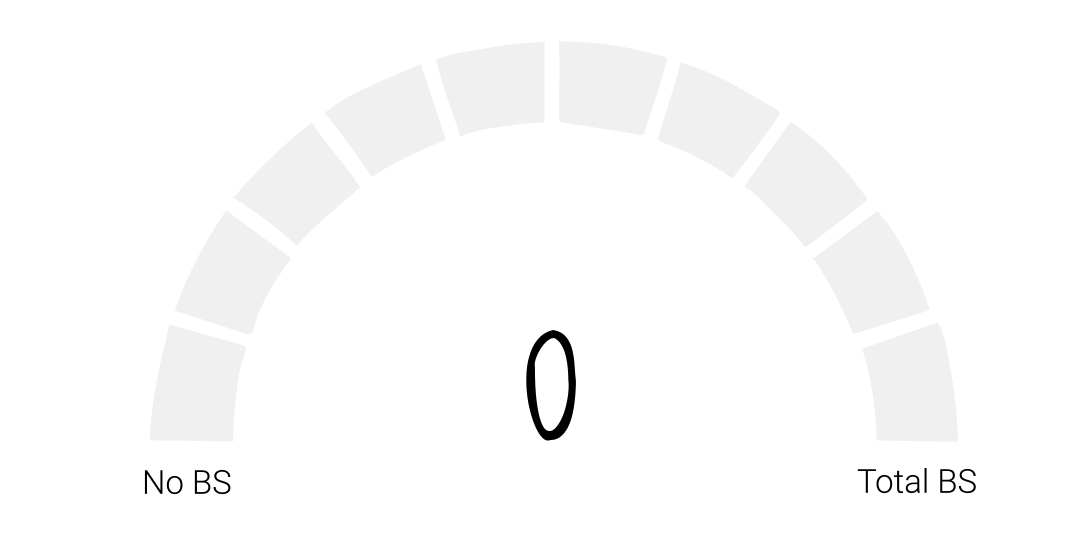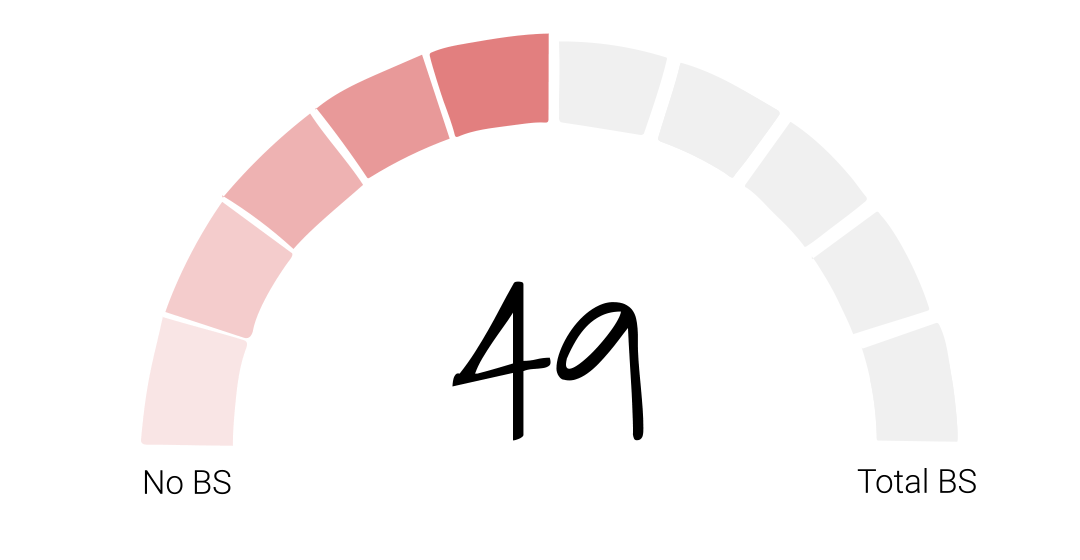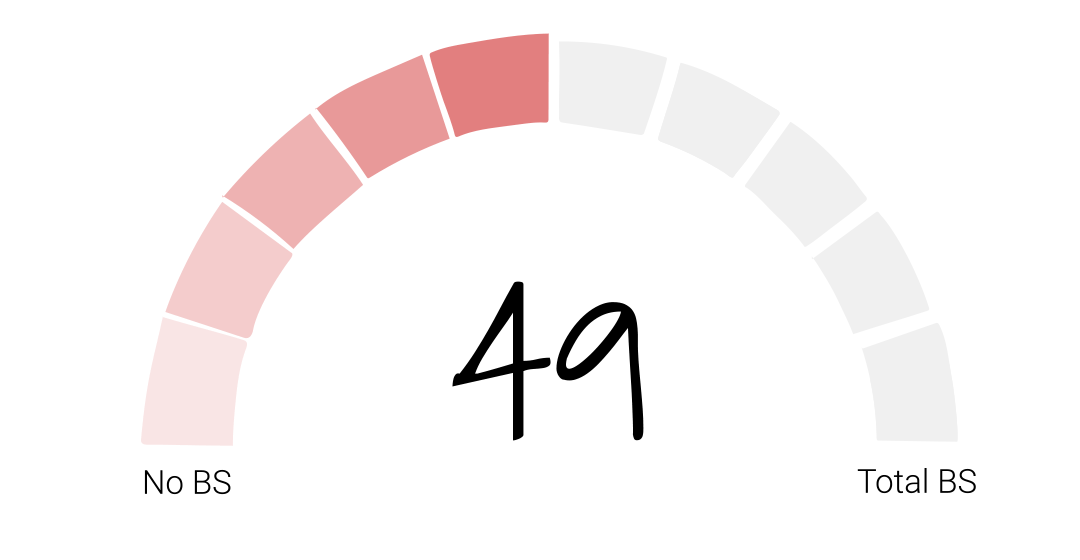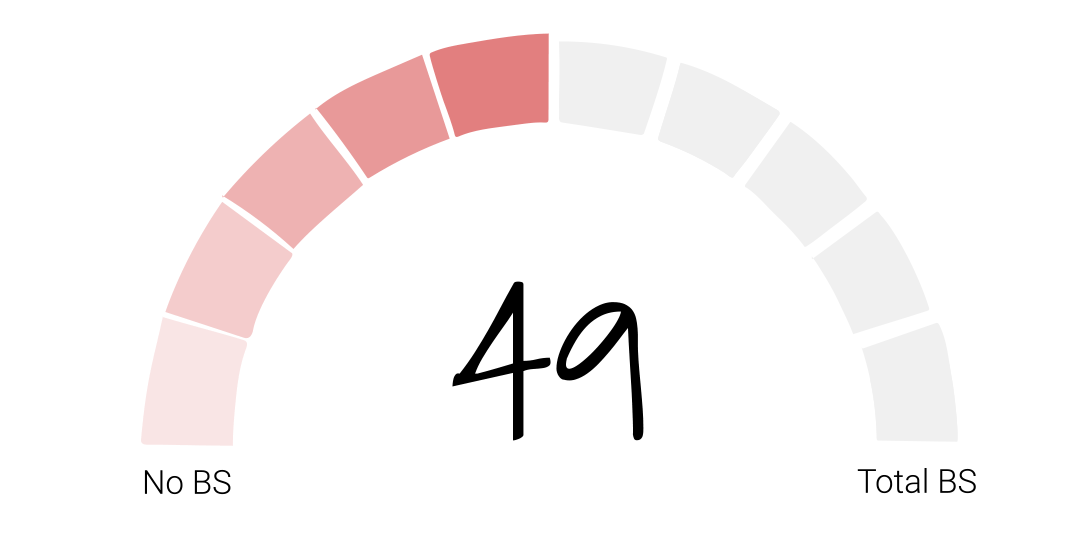
In our initial investigation into Juul, we concluded that the e-cigarette company was a bullshitter worthy of a BS score of 49. Their stated purpose – to reduce the harm of cigarettes by giving smokers a healthier alternative – had been overshadowed by their decision to market directly to young people which led to a whole new generation addicted to nicotine – their legacy, as former FDA Commissioner Gottlieb called it.
Join us as we welcome back a season one guest to decipher what this means for Juul and just what the heck is going on at the FDA.
TY MONTAGUE (VO)
Welcome to Calling Bullshit, the podcast about purpose-washing…the gap between what companies say they stand for and what they actually do — and what they would need to change to practice what they preach.
I’m your host, Ty Montague. I’ve spent over a decade helping companies define what they stand for — their purpose — and helped them to use that purpose to drive transformation throughout their business.
Unfortunately, at a lot of organizations today, there’s still a pretty wide gap between word and deed. That gap has a name: we call it Bullshit.
But — and this is important — we believe that Bullshit is a treatable condition. So when the BS detector lights up, we’re going to explore things that a company should do to fix it.
TY MONTAGUE
Okay, folks. I am very excited to introduce Lauren Etter for her second time on the show. Lauren, thank you for being here, and welcome back to Calling Bullshit.
LAUREN ETTER
Thanks for having me, Ty. Great to be here.
TY MONTAGUE
So just for our audience quickly, those who haven’t listened to the Juul episode, it would be great to hear just a little bit about your background.
LAUREN ETTER
So I’m an investigative reporter at Bloomberg news. I recently wrote a book called The Devil’s Playbook about Juul, and I’ve been a reporter for many years.
TY MONTAGUE
So yeah, you were here the first time to talk about Juul and it’s a story in which both Juul and the FDA figured prominently and both of them Juul and the FDA have been back in the news again lately. Can you walk us through what happened?
LAUREN ETTER
Absolutely. So as your listeners might recall, Juul has been in the crosshairs of the federal government and opponents of its product for many years. So since it hit the market in 2015, the product came under a lot of scrutiny, both from regulators and from opponents who blamed the company for essentially sparking a new youth nicotine epidemic so that was several years ago, and fast forward.
The FDA has been waning, whether or not to allow Juul to continue selling its product in the US market. So for the past two years, the agency has been reviewing a very voluminous application by Juul that was essentially the company’s effort and way to say that this product deserves to be on the market.
It meets the regulatory standards. Essentially saying that it’s a benefit to the public health and it’s going to ultimately help adult smokers quit smoking. So this has been going on for a long time. And as you might recall, Juul reached unicorn status in Silicon Valley, it was a multi-billion dollar company that had essentially garnered an investment from Altria, the maker of Marlboro cigarettes and it was kind of a Silicon Valley darling for a while, even though it had gotten all of this bad press and negative reviews because of its public health problem.
TY MONTAGUE
Yeah. And I would say some, a lot of that criticism was, I think in many ways fair. In the first episode, you and I agreed, and, and I, I learned this from your book that there was no reason that Juul had to get negative press or feedback, but the fatal flaw was the way that they marketed the product, that the product itself would’ve been great for smoking cessation, getting people to quit smoking. But smokers are generally older and the marketing of Juul, clearly, at least in the early going targeted young people.
LAUREN ETTER
That’s exactly right. So the founders of Juul created this product, ostensibly, to be an alternative to cigarette smoking. And so that was what they said, but when the product launched, initially, they introduced the product to the world with a very splashy marketing campaign with images of very youthful models in Times Square and all over social media, and all of a sudden you saw a huge number of middle schoolers, high schoolers, starting to not only use Juul, but become highly addicted to it. And that’s the key thing to remember about Juul. It is a 5% nicotine solution, which in each tiny little pod the amount of nicotine is equivalent to that in a pack of Marlboro Reds, for example. So it’s a highly addictive product.
Young people with developing brains are uniquely predisposed to becoming addicted to nicotine, and it’s an extremely difficult habit to kick. And this came on the heels of decades of actual public health successes with youth smoking. Teen smoking rates had been declining year after year after year, a real public health coup, and then suddenly vaping and Juul come along and kind of blow that up and create a new public health controversy.
TY MONTAGUE
Right? Exactly. So then the FDA has been considering this and they rendered their opinion. What did they have to say to Juul?
LAUREN ETTER
So it was a really surprising decision. Essentially the FDA many years ago set out a regulatory framework and that framework stipulated that any new nicotine product must go through a rigorous scientific review process by the agency before it was allowed to remain for sale, essentially.
So Juul has stood by its commitment and claims that this product will help adult smokers. So they put together a gigantic package of information and people asked me even a year ago, you know, will Juul get the FDA authorization. And I really was under the impression that they likely would. I always said I would be very surprised if the FDA denied their authorization for a number of reasons.
Number one, the United States is trying to move smokers away from cigarettes in general. They’re taking steps to dial down the amount of nicotine in combustible cigarettes. The Biden administration has moved in the direction of banning menthol cigarettes. So the whole idea here is there’s a bigger harm reduction kind of framework that the US is looking at right now, which would be let’s figure out ways to migrate adult smokers off of deadly cigarettes and onto something that is potentially less deadly. So there’s a continuum of risk there. Smokers can quit cold Turkey. They could use a pharmaceutical drug like Chantix. They could use the patch. And then there’s also these other products now that are more popular like e-cigarettes.
So the FDA has in a way kind of embraced this idea that there should be alternatives for smokers, adult smokers, including e-cigarettes. So given that whole context and backdrop. And knowing that Juul is the single largest maker of e-cigarettes in the United States. I thought it would’ve been surprising if the FDA would move to not authorize the product, not only that, but Juul is the only product that Juul makes essentially. So if the FDA were to take them off the market, they would go bankrupt. I didn’t.
TY MONTAGUE
Kills the company,
LAUREN ETTER
Yeah, US a federal agency would be reluctant to do that.
However, so fast forward in June of this year, the FDA’s long-awaited decision and much-delayed decision by the way, finally came down and they said, indeed, that they were going to not authorize Juul to remain on the market. In essence, banning the product. it was a huge decision. It really took everybody by surprise.
Even took people at Juul by surprise. And it has some very large, and important ramifications.
TY MONTAGUE
Right. And my understanding is there’s been a stay. Right. That Juul petitioned the court to say, Hey, don’t make us take the product off tomorrow morning. And that was granted. Is that correct?
LAUREN ETTER
Yeah. So it ended up in a very unusual kind of space. So what happened was the FDA makes this big splashy announcement, people who are supporters of Juul, Juul, people, themselves kind of panic and accuse the FDA of not following the science of kind of veering outside their lane as a regulator and arriving at this decision.
And so a couple of things should be pointed out. So one, the reason that the FDA gave for denying their authorization was surprising. You might have thought that it would’ve been “Well Juul, you caused this youth epidemic. We’re gonna punish you and we’re not authorizing your product because your product is not in furtherance of the public health.
However, that’s not what the agency said. The agency actually said, “we are not authorizing the product because Juul did not provide enough information to show that their products were not harmful.” They said that they did not provide enough science to back up the claim that it wouldn’t be damaging to human health. In particular, they actually focused on the pod and said that the pod could be leaching and Juul didn’t prove that it wasn’t leaching and wasn’t causing human health concerns. So it was a very unusual decision that was surprising in a number of ways.
So not surprisingly Juul immediately sued. They basically went to the U.S. Court and asked for an emergency stay, which would basically say, look, you can’t take our product off the market immediately. you have to consider all these other factors, including the fact that we Juul, we don’t believe that the FDA did a thorough and fair review of all of the science that the company provided.
So the, the court granted that emergency stay and then shortly thereafter, the FDA.
TY MONTAGUE
Walked it back.
LAUREN ETTER
They walked it back and said, actually, we’re taking the application back and we’re going to continue reviewing it in light of all of this information that Juul had brought forward.
TY MONTAGUE
Yeah, very, very surprising and complicated. The, the, the language they used, “the agency has determined that there are scientific issues, unique to the Juul application, that warrant additional review” set off alarm bells in my head.
I don’t know whether they set off any in yours, but I’m trying to understand what that means, you know, because I immediately went well there was, there has been some coverage about the pressure that the FDA has been under from Congress to do something about Juul. And so it made me wonder, like, is the fix in, at the FDA? Is that why they’re walking it back because somebody got to somebody, or do you think that legitimately there are scientific issues that, that deserve to be reviewed?
LAUREN ETTER
I mean, I think there would have to be a lot going on inside the FDA to reach the conclusion that, you know, the fix was in. I mean, certainly there are people who believe that, I’m not one of them. I think that the agency in general does and was acting in good faith. It’s just more than anything to me, it indicates maybe, the agency has been overwhelmed with a number of applications.
Maybe they’re overworked, it’s it just, it, it it’s unusual. Doesn’t quite make sense to me. But I, I don’t necessarily think the fix was in. I think that they did have legitimate concerns and you must assume that the agency gave a thorough review to their product. It was, it was being reviewed for two years.
TY MONTAGUE
Yeah. I agree. I apologize for interrupting you, but, there’s something about this story that just bothers me. First of all, you know, not to beat up on, on the FDA, but it took them a long time. They let Juul market to young people openly with impunity for many, many years and, and genuinely have now addicted a new generation of young people to nicotine. Then they’ve reviewed this decision for two years and they come out with this very strong response for some people, maybe too far for others, but, finally, you know, there’s some, some action on the part of the FDA and then they walk it back. Like that just seems bizarre to me.
LAUREN ETTER
Well, I, I would agree that the, the way that the FDA handled the Juul matter does not engender confidence in the agency. That’s for sure. It raises a lot of questions about how they could have botched this, why they didn’t review every piece of evidence.
Why, especially after having it under review for so many years, did they miss this key that Juul claims that they missed? I, I it’s, it’s absolutely unusual and unsettling. I just don’t have the answer as to why exactly the agency is doing it other than what they’ve articulated in their public releases and in court filings.
TY MONTAGUE
Right. I’d be curious just to hear whether you personally think this, this ban is gonna hold or whether you think they’re gonna walk away from it, like, do you, what do you think the future looks like?
LAUREN ETTER
It’s just hard to know this, this issue now lives in a very technical administrative legal world. It’s all about the administrative procedure act and whether or not the agency upheld its end of the bargain, essentially to do an unbiased review. And what I will say is that Juul is certainly ready and capable to amass a legal defense. This is what the industry does and what they have been essentially experts at for the past five decades or more. They have a bunch of lawyers they’re ready to litigate. So this is not gonna get resolved anytime soon and it’s hard for me without having a crystal ball to know exactly what the agency’s ultimately gonna do.
But in the meantime, it’s a status quo. The product is still available. Opponents of the product are still out there really upset that the agency has done this. I mean, it’s, it’s an issue that doesn’t seem to want to go away. That’s not gonna go away anytime soon.
TY MONTAGUE
I know as we said in the original program, if they had not marketed it to youth, initially, if they had gone after actual smokers and tried to get them to switch it, would’ve been a great service to the world, like, honestly, and it’s just a shame that they didn’t do that and now things continue to be sort of vague and messy.
LAUREN ETTER
Well, and I would be remiss to not point out the reason that that happened is because this was a Silicon Valley startup that chased one thing only, and that was hyper-growth. This was a company that wanted to attain unicorn status that eventually did. And there were a lot of investors that made a lot of money.
Had they chosen to go a more plodding kind of responsible path. They might have en ended up making Juul, but Juul might not have ever seen the light of day because no investor in Silicon valley would’ve invested in a company that said, Hey, 10 years from now, once we have our approval or 15 years from now once we have our approval, we’ll have a, this great product that people are gonna love. So I fault silicon valley and the investors on that side.
TY MONTAGUE
And it’s interesting because the Silicon Valley solved a really profound technological challenge in creating Juul in the first place. One that the cigarette companies have been trying to solve for years and failed. And so, in a way, It had to come from the Silicon Valley in order to get out of this sort of group think of the traditional tobacco companies, but it putting it in Silicon Valley’s hands, created a whole host of new problems.
MUSIC: “In Passage” by Migration
It’s such an interesting point. So Lauren, thank you so much for coming back on the show. It was great to see you. We appreciate you being here.
LAUREN ETTER
Thanks for having me.
TY MONTAGUE (VO)
Folks, after looking at all of the new facts, I’m going to keep Juul’s BS score at 49. Juul has taken none of the actions we talked about in the episode in Season 1 and the product is still available in convenience stores across the country and around the world within easy reach of young consumers. So whether or not they sincerely want vaping to be a path to smoking cessation remains to be seen. And if the last two years have been any indication, we’ll be waiting awhile to find out.
However, in this new chapter of the Juul story, it’s the FDA’s actions that perplex me the most. Taking years to make a decision on Juul, then making a strong ruling against it, and then immediately walking that decision back just doesn’t add up. It made me wonder… is there bullshit to be found in the FDA?. Stay tuned, because we’re about to find out.
Thanks for joining me on this BS update episode and a special thanks to Lauren Etter, author of The Devil’s Playbook: Big Tobacco, Juul, and the Addiction of a New Generation.
If you think Juul’s BS score of 49 needs revision, let us know on our site calllingbullshitpodcast.com or on one of our socials @callingbullshitpodcast. And friends, I’d like to ask for your help. If you enjoy the Calling Bullshit podcast, please take a second to rate and review us on Apple podcasts or your preferred platform.
Thanks to our production team. Hannah Beal, Amanda Ginsburg, DS Moss, Haley Paskalides, Parker Silzer, and Basil Soper.
Calling Bullshit was created by co:collective and is hosted by me, Ty Montague. Thanks for listening.
Tagged as: Juul.
Calling Bullsh!t September 23, 2022
We’re back. Join us as we ask weight loss app Noom to step on the scale, analyze Robinhood’s aim to “democratize finance for all” and consult with some real experts […]
Code Herb on October 23, 2022
Thanks for an explanation. All ingenious is simple.