
Unilever: Making Sustainable Living Commonplace
Unilever’s purpose is “to make sustainable living commonplace.” The sheer size of this company makes that an especially difficult task. But it also means that when they do meet their […]
 play_arrow
play_arrow
Whistleblowers: A very special kind of bs detector Calling Bullsh!t
 play_arrow
play_arrow
McKinsey: Something to hide? Calling Bullsh!t
 play_arrow
play_arrow
Web3: A more humane, egalitarian, and decentralized internet? Calling Bullsh!t

 play_arrow
play_arrow
FDA: Is there something rotten here?
Calling Bullsh!t

Author of Fixing Food: An FDA Insider Unravels the Myths and the Solutions


Author of The Devil’s Playbook: Big Tobacco, Juul, and the Addiction of a New Generation.

Stated Purpose: Today as in the past, the FDA strives above all else to safeguard the health and well-being of the American people.
To say that the FDA is a complex institution with vast responsibilities would be an understatement. Is this bureaucratic behemoth truly protecting us from companies that would otherwise put profit before the public’s safety? The stakes couldn’t be higher for the American people. Tune in to find out.
“I said “I’m not an economic prostitute” And he said “Well, then you’re fired.”
– Richard Williams
MUSIC: “In Passage” by Migration
GAIL VAN NORMAN
Overall the FDA has had a number of spectacular hits and pretty notable failures.
LAUREN ETTER
And it, it is worth asking how good of a job is the agency doing and are they living up to their mandate to protect the public health?
RICHARD WILLIAMS
My boss at the time said, Richard, we don’t have to solve problems. All we have to do is appear to solve problems.
TY MONTAGUE (VO)
Welcome to Calling Bullshit, the podcast about purpose-washing — the gap between what an organization says they stand for and what they actually do — and what they would need to change to practice what they preach.
I’m your host, TY MONTAGUE. I’ve spent over a decade helping organizations define what they stand for — their purpose — and helped them to use that purpose to drive transformation throughout their business.
Unfortunately, at a lot of institutions today, there’s still a pretty wide gap between word and deed. That gap has a name: Bullshit.
But — and this is important – bullshit is a treatable condition. So when our bullshit detector lights up, we’re going to explore everything the organization should do to fix it.
TY MONTAGUE (VO)
There was a time in the not-too-distant past when cocaine was marketed to children, and literal snake oil was sold as medicine! A time when sawdust was put into food as fillers and when rats were ground up with beef. And it was all perfectly legal. Food and drugs were sold in a completely unregulated market – until President Theodore Roosevelt signed into law The Food and Drugs Act of 1906, giving the executive branch the power to regulate foods and drugs and thus creating what we now call the FDA.
Today, the Food and Drug Administration has expansive jurisdiction, regulating things ranging from microwave ovens to catnip to ibuprofen and beyond. Way beyond. Chew on this – The FDA regulates twenty cents of every dollar you spend.
To cover such a broad waterfront, they employ 18,000 people that work across several inter-agencies including the centers for Drugs, Biologics, Devices, Veterinary Medicine, Food Safety & Nutrition, and Tobacco.
Their formal purpose is a few paragraphs long, but on the homepage of their website, they have a phrase that nicely captures the spirit of it. And it reads – “today as in the past, the FDA strives above all else to safeguard the health and wellbeing of the American people.”
That is a monumentally important purpose – one in which the stakes – for all of us – are high. However, if you’ve been listening to the news lately it does seem like the FDA is falling pretty short of living up to it.
[SOT] Speaking about the epidemic of youth use of e-cigarettes, in retrospect the FDA should have acted sooner.
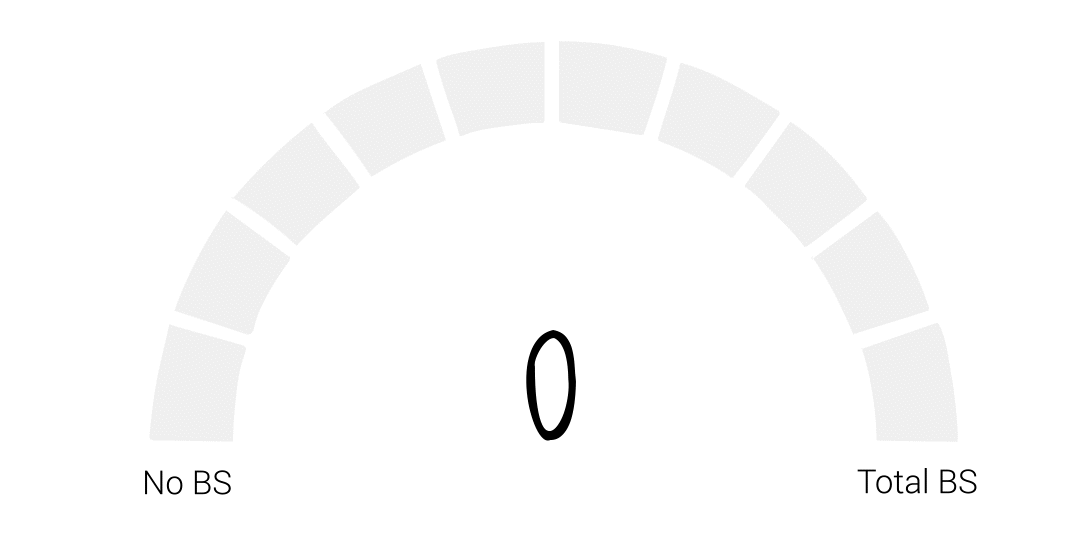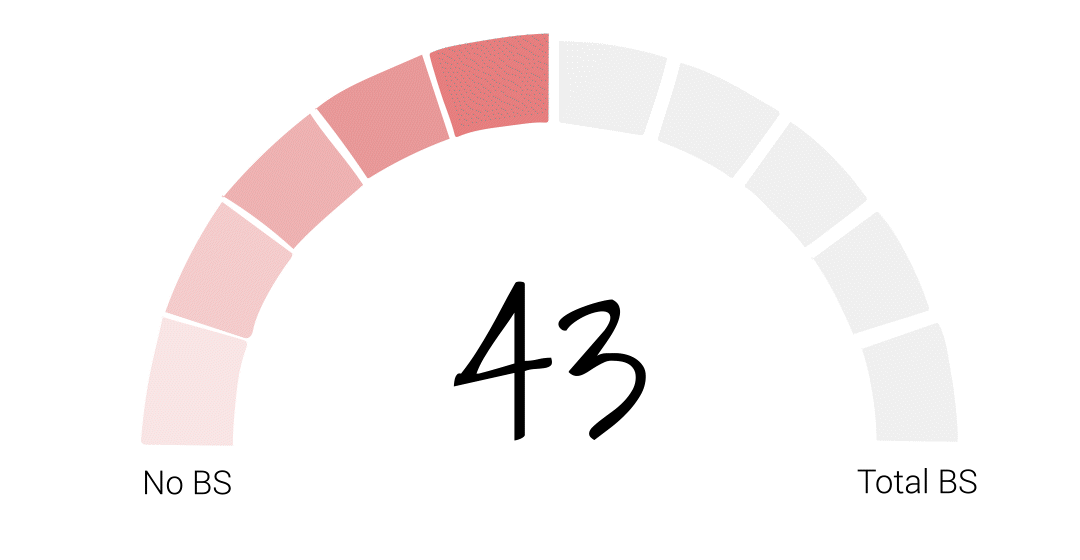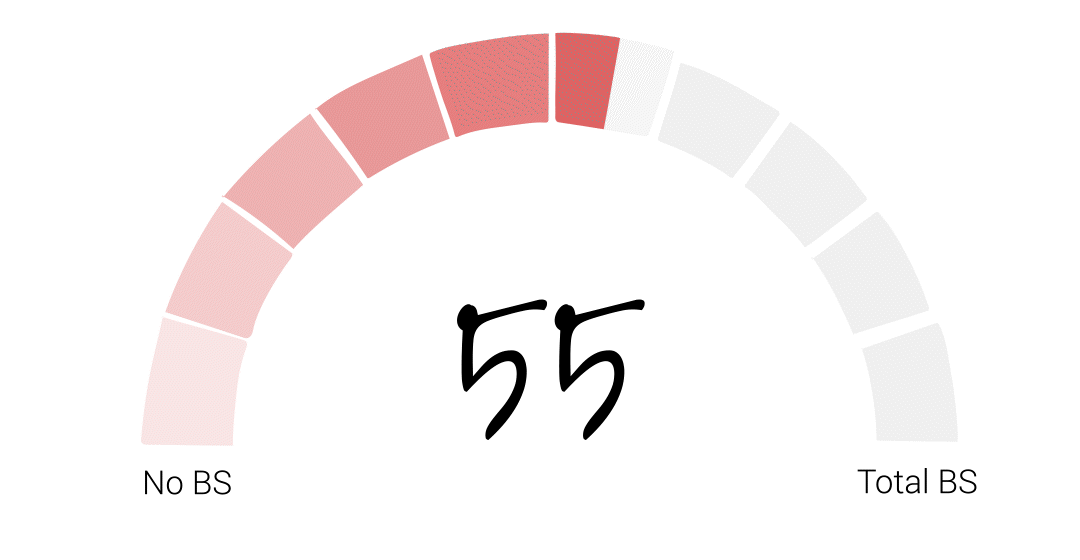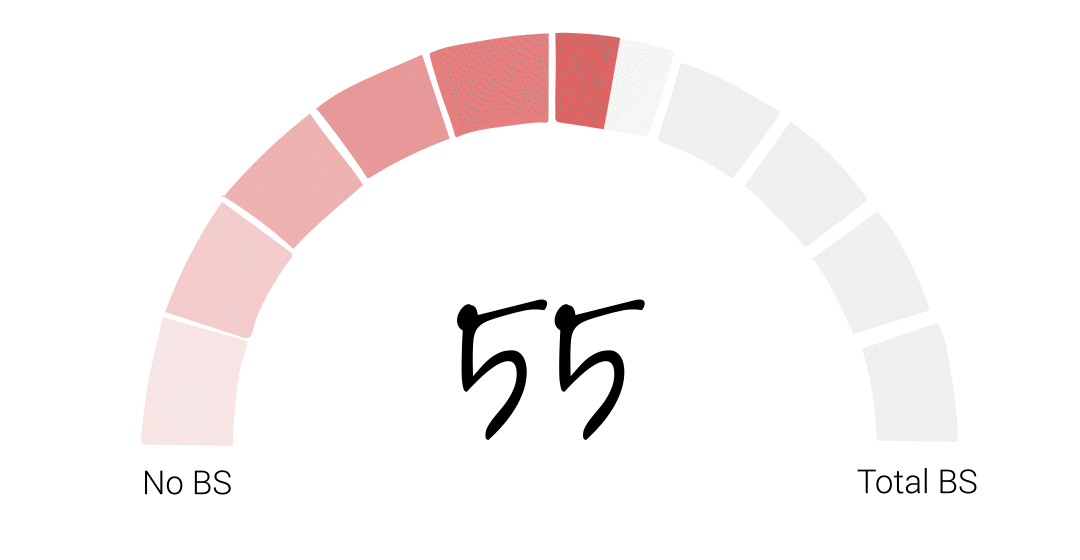
[SOT] Nationwide, 43% of baby formula stock is gone.
[SOT] Part of the blame for a lot of these shortages rests at the feet of the FDA.
[SOT SENATOR HASSAN] in 1995, the FDA first approved Oxycontin. Since then, more than 500,000 people have died from opioid-related overdoses.
TY MONTAGUE (VO)
We’ve actually encountered the FDA twice before. First when discussing their role in regulating Juul E-cigarettes, and again while researching Purdue Pharma for a potential episode, which we ultimately didn’t produce because, well, there is absolutely nothing redeemable about Purdue or the Sackler family.
The FDA was certainly a character in the first Juul episode. But when we checked in on the company again this season, it was clear that the FDA shared a lot more of the blame for the damage caused by Juul’s unregulated e-cigarettes.
And so, we decided to aim our bs detectors at the US Food and Drug Administration – starting with picking up the thread of the strange case of big tobacco, vaping, and JUUL…
TY MONTAGUE
Okay folks. I am very excited to introduce LAUREN ETTER for her second time on the show. Lauren, thank you for being here, and welcome back to Calling Bullshit.
LAUREN ETTER
Thanks for having me, Ty. Great to be here.
TY MONTAGUE (VO)
Lauren is the author of The Devil’s Playbook: Big Tobacco, Juul, and the Addiction of a New Generation.
But before we continue the conversation with Lauren, let’s catch you up on the escalating story of the regulator and the company with a BS Roundtable recap:
MUSIC: ROUND TABLE RECAP THEME
Person 1
Juul Labs launched its Juul e-cigarette as a smoking cessation device in 2015.
Person 2
It was actually effective and had the potential to help smokers get off cigarettes.
Person 1
But Juul was funded by venture capitalists with a growth-at-all-costs mindset.
Person 3
Which led them to target kids. The demographic most susceptible to picking up a smoking habit..
Person 2
…thus sparking a new youth nicotine epidemic
Person 3
Total BS. But at the time e-cigarettes weren’t regulated.
Person 1
Finally, in 2016, the FDA began regulating e-cigarettes as tobacco products and requiring manufacturers to submit applications to be on the market.
Person 2
And guess what? Juul Labs didn’t get around to submitting their application to the FDA until July 30th, 2020, all the while they were still selling Juul.
Person 1
More BS
Person 3
You would think the story ends here. But it doesn’t. It then took the FDA two more years to review Juul’s application.
Person 2:
All the while – Juuls are still being sold.
Person 3
Finally, in June of 22 the FDA announced their decision to deny Juul Lab’s application- essentially banning the e-cigarette. Their reasoning – Juul did not provide enough scientific data to show that their products were not harmful.
Person 1
Not surprisingly, Juul Labs immediately sued and within a few days was granted an emergency stay allowing them to keep their product on the market.
Person 2
And NOW the FDA has walked back their decision and is currently re-reviewing the application leaving Juul e-cigarettes – you guessed it – still on the market. And that’s your roundtable recap.
MUSIC: END ROUND TABLE RECAP THEME
TY MONTAGUE
The FDA website says “today as in the past, the FDA strives above all else to safeguard the health and wellbeing of the American people.”
So, first I just wanted to ask you, how do you think they’re doing at meeting their mission or achieving their purpose?
LAUREN ETTER
I would say just in general, that with tobacco, for many years until the early 2000s, the agency didn’t have any jurisdiction over the tobacco industry. It was a completely unregulated market and it was only after the Master Settlement Agreement and after the tobacco companies had gotten crushed and that whole ordeal, when, you know, for years, Congress tried to figure out how to regulate tobacco. The Supreme court, even at one point said that they didn’t have jurisdiction. So this has been an issue like where should the tobacco industry sit? Does it make sense for the FDA to regulate this industry?
But for years, the industry was totally unregulated. So I think that there’s a lot of questions worthy of asking about the FDA, and certainly about their role in the regulation of tobacco.
TY MONTAGUE
You may remember that we score organizations on their level of BS. Would you be comfortable giving the FDA a score even as it relates to the Juul situation of zero to 100 in terms of gaps between word and deed.
LAUREN ETTER
So if you look at what the agency is doing, In the tobacco, the overall kind of tobacco space and their efforts to implement a harm reduction framework they’re actually making strides. my issue is I don’t feel like the FDA has enough resources to police the kind of illicit sale of the product to keep it out of the hands of the youth.
They’re just a very underfunded agency, especially the center for tobacco products. And so if you’re looking at the twin problems of adult smoking and youth nicotine addiction, I feel like they’re, they are focusing quite a bit on the adult smoking issue. and then the youth , nicotine addiction issue, I don’t know if I have enough confidence that they’re going to be able to ultimately keep this highly addictive product outta the hands of kids, which is ultimately the goal to stop a new generation from becoming addicted. So, can I give them a score? Um, I just don’t know if I feel comfortable settling on a, on a number.
TY MONTAGUE
Yeah, that’s okay. But, it’s, it’s definitely not the best number zero, right? they’re bumbling their way through this. And, let’s just say with best of intention, right? They have failed to protect America’s youth so far from vaping, which is at the end of the day, an extremely efficient delivery system for what ought to be a controlled substance in my view.
LAUREN ETTER
Right. But the FDA is constantly barraged with this flood of new products. And they’re constantly on their heels, responding to these, like, rapidly innovating markets that makes it very difficult for them to contain this, again, highly addictive product that’s being sold at seven elevens and, you know, corner stores around the country.
Not all of them have the greatest ID checking abilities. So I admit it’s a very difficult task for them to do. I think it should be one of the most important tasks that they undertake. And I know that they’re taking it seriously. I’m just concerned that they might not have enough resources to adequately keep these highly addictive products outta the hands of young people.
TY MONTAGUE (VO)
Is the FDA’s lack of resources the reason they’ve fumbled through regulating e-cigarettes and Juul in particular? Lauren thinks so – or at least it’s a significant part of it. And although she deferred giving them a BS Score, she does believe the FDA is making strides.
No doubt it’s an incredibly challenging undertaking, but if your purpose is to safeguard the health of the American people and a new generation gets addicted to nicotine on your watch, it’s definitely a harbinger of bullshit.
But maybe the Center for Tobacco Products is an outlier? Considering the FDA covers so much ground, I’m going to reserve judgment and see what’s cooking at their second biggest inter-agency – the Center of Food Safety and Applied Nutrition.
RICHARD WILLIAMS
with food specific, we’re trying to do a little more, because we don’t want to just make food safe. We want to give consumers information that help them to choose a healthier diet.
TY MONTAGUE (VO)
Richard Williams worked at the FDA for nearly three decades, and he left the Center for Food Safety and Applied Nutrition with a bad taste in his mouth.
RICHARD WILLIAMS
I think the big thing was as I approached the end of my 27 years, It occurred to me that most of what we had done had not succeeded. And so the more I thought about it, the more I thought, well, what actually happened, what went wrong? And I thought maybe if I could write a book about it, we can start changing things and, and actually solving things for consumers.
TY MONTAGUE (VO)
Richard’s book, Fixing Food: An FDA Insider Unravels the Myth and the Solutions is an eye opening account of his tenure at the organization, beginning when he was hired as their very first economic analyst.
RICHARD WILLIAMS
You can blame it on President Carter. Uh, President Carter was the first one who said, we, we need some economic thinking in these particularly social regulations.
Presidents began insisting that we do these things and that we take, take them into account. so we can make better decisions.
TY MONTAGUE
Let’s talk about your job and some of the eye opening, parts of your book, because, you, like walked into that job with the best of intention you wanted to help the, FDA achieve its mission of making people safer.
One of the first assignments that you had was to do a cost benefit analysis on lead acetate in men’s hair dye. And I wondered if you would tell us that story?
RICHARD WILLIAMS
Sure. That was the first one just assigned to me. all I had to go by was the executive order, and what I knew from, from, being an economist. So I jumped into it and found out that this is, was basically the thing that you put in your hair. And over time it takes the gray away.
TY MONTAGUE (VO)
Richard wanted to know if using lead acetate could cause skin cancer. And after a deep dive into the toxicology, he found the risk was near zero. Thus, there was no BENEFIT to banning the drug. There was, however, a high COST to taking it off the market- it was the only product of its kind available. So without it, gray-haired men would have no alternatives. (Other than living with the fact that their hair was gray).
RICHARD WILLIAMS
I turned in my analysis. Didn’t think a thing about it until a couple of weeks later, a young woman had come down from the center director’s office and she said, this is great. Now we need you to do the other one.
And I said, what other one? She said, well, you said the benefits were lower than the cost. and if we decide, not to do anything about lead acetate that’ll work, but we want you to do one that shows the exact opposite that the benefits exceed the cost, that it does cause cancer.
And I’m like, well, no, that wouldn’t be honest. I’m not going to do that. And she said, you don’t understand, this is an order, you know, from the sixth floor. And I said, no, I’m not going to do that. I didn’t hear anything more about it. A few weeks later, I was in our first, my first training class where I was getting my introduction to FDA, and the deputy center director was in there, and at lunch, I went up and I said, you know, a funny thing happened to me. I. And I explained the situation and he said that order came from me and you’re going to do it, or you’re going to be fired. And I said, something really stupid. I just made this up on the spot. I said I’m not an economic prostitute. And he said, then you are fired and you are going to be leaving this agency.
TY MONTAGUE
Good on you.
RICHARD WILLIAMS
So I went back to my office. I’m like, should I start packing up my stuff? I didn’t know. And then nothing ever happened. He apparently decided it would be a bad idea to fire me over that of thing. But that was just the first time I was threatened with being fired.
TY MONTAGUE
I mean, good on you that you did not cave to that pressure, but it sounds like that this was not an isolated occurrence. Were there other instances where you were asked to essentially compromise your integrity?
RICHARD WILLIAMS
For 27 years, there were other instances. It was, it was com it never stopped.
TY MONTAGUE
That is incomprehensible to me, really?
RICHARD WILLIAMS
It is.
TY MONTAGUE
That is terrible.
RICHARD WILLIAMS
For the first time in my life, I decided to become political with a small P like a, a bureaucrat politician, which you have to be,
TY MONTAGUE
Yeah, sadly. Well, so let’s, let’s follow that thread a little bit because, I assume that let’s assume the best of people. I assume that people who were feeling the pressure above you in the organization were feeling it from other entities.
You know, in your book, you go into the FDA spending a lot of energy, responding to influence from just an incredible variety of stakeholders. There’s you know, the executive branch, there’s Congress, there’s courts, there’s press, private industry activists, academics.
Can you help us understand the FDA’s relationship with some of those stakeholders?
RICHARD WILLIAMS
Sure. I mean, you know, first of all, you have to start understanding one thing FDA’s been around since 1906 and they have continued to accumulate, literally power such that one author said that in the 20th century, there was no regulatory agency in the world that was more powerful than FDA. and that’s because they’re very good at, at what they do.
So if you think about it, they work for the president and the presidents, are supposed to oversee them. But every president Republican and Democrat has said, it’s nearly impossible to control the administrative state. That is all the agencies that, that they oversee. There are hundreds of thousands of employees.
They all have their own little agendas and it’s very, very difficult for the presidents to do anything. So that’s a problem. Congress, they just don’t oversee FDA. They’re afraid of FDA. so there’s not a lot of oversight there.
TY MONTAGUE
Interesting. Why is Congress afraid of the FDA?
RICHARD WILLIAMS
Well, FDA has very complex issues. Very hard for Congress people are really busy, you know, with lots and lots of things, mostly getting reelected and raising money. They don’t have a lot of time and their staffs aren’t very good at overseeing these complex issues.
I get it, but it means that FDA’s kind of more free to do what they want. They hold Congress at bay, the courts have up until recently and generally will give them deference to how they interpret their statutes. Then they have other people. Academics they generally give grants to, that kind of buys them off. And then when they go in to Congress to ask, you know, which they do every year for more money, they actually have large food firms going in testifying saying, yeah, FDA should get more money. There’s a reason for that. Large firms usually get regulations that are easy for them to comply with, but hard for small firms.
So it puts their smaller competitors at a disadvantage.
TY MONTAGUE
I want to go into that issue a little bit later, the issue of big companies versus small companies, because that, that feels like an important topic. But before we go there, let’s talk about one other constituency– consumers– and where they rank that hierarchy.
RICHARD WILLIAMS
I think consumers and small businesses both are at the bottom. when I first came into FDA you know, I truly believed in what we were trying to do. I thought these are important issues and over time I found out, well initially when I got there, I was told this, that, and this actually happened with the lead acetate rule.
This rule won’t do anything. Why are we doing it? And my boss at the time said, Richard, we don’t have to solve problems. All we have to do is appear to solve problems. We have to do something about it, and that never left me. And I noticed more and more. That was the case. Once we passed a regulation, no matter what the regulation did, it didn’t matter. It looked like we had addressed the problem.that, and that leaves consumers. Public health in, in, in last place, if you will.
TY MONTAGUE
What a mess. Okay. So. let’s talk about some of the, you know, in your view, the biggest and most dangerous problems that face consumers today that the FDA is responsible for. And we can live within the domain of food, but feel free also to talk about, stuff outside of food.
RICHARD WILLIAMS
Yeah. well, I, I do think it’s obesity. I, I think that is probably the number one. And it’s going to get worse. you know, there was a Harvard study that projected that by the end of this decade, which is only eight years away, half of this country will be obese.
FDA has a role in that with food labeling. Food labeling has not worked. It’s not going to work. It’s really not going to be the answer. Putting calories on foods hasn’t really changed anyone’s behavior as far as we can tell.
TY MONTAGUE
Right. Sticking with obesity and I don’t think you, you made this connection overtly in the book, but one of the impressions that I’ve gotten both from, you know, what we’ve learned from your book, also other research that we’ve done is that. Intended or not the combination of the industrialized food system where people eat a bad diet and combine that with a for-profit healthcare system that then profits from the multiple diseases that result from things like obesity is kind of the perfect dystopian partnership.
What do you think about that first? And, and do you think the FDA has played a role in that?
RICHARD WILLIAMS
I don’t personally think that is the exact issue. I, I think it is an issue. I think certainly, food companies, like every other company in, in this country are there to make a profit. The problem is, we have no idea what a healthy diet looks like. because nutrition science is the worst science that we have.
And so we’ve got people out there hocking books, you know, on diets some say, well, you gotta have a high carb diet. Some say you have to have a low carb diet, high, fat diet, low fat diet on and on and on. And the truth the matter is one we don’t know. And two, what we’re beginning to find out is that one diet probably isn’t right for everyone. Different people with different genetic backgrounds, different underlying health conditions probably need different diets. So I think in my mind, a bigger problem, and this is why, basically companies can sell whatever they want is cuz we just don’t know enough, yet, because the science is so bad.
TY MONTAGUE
Well, that, that, that’s a good point. I think, to pivot to another aspect that you touch on in your book, that you believe that entrepreneurs are solving many problems that the FDA can’t or won’t. Can you talk more about that?
RICHARD WILLIAMS
FDA has recently announced- they’re gonna start looking at technologies. So the first thing that they’re gonna do- and this is gonna be tremendously helpful, if we can get it done- is using blockchain, the same thing that you use for cryptocurrency, in order to start recalling things faster.
TY MONTAGUE (VO)
For example, this technology could – in theory- reduce the time it takes to trace the origin of a contaminated food outbreak from two weeks down to two seconds.
RICHARD WILLIAMS
So that does several things. One, if you can trace things back very quickly, you can find out what the root cause was in other words what actually went wrong that caused this problem.
More importantly, it gets bad products off the market more quickly, and it also doesn’t basically indict everybody.
TY MONTAGUE
Right. Yes. which sort of leads to something that you’ve hinted at already, in this interview, which is that the playing field is not necessarily level between companies and big companies. Can you talk more about that?
RICHARD WILLIAMS
So there’s a law, the regulatory flexibility access we have to take into account, you know, what the impacts of these things are on small producers and if we can, to give them some kind of relief.
Given the fact that the benefits weren’t that great, I said, well, at least let’s exempt some of these small firms or make the requirements easier on them. We didn’t do that, of course all the large firms didn’t want to give small firms a break, because they say it’s an unlevel playing field. They have much more, influence over FDA- and this is true in any regulatory agency- than the small firms do. So the small firms get driven out of business.
And I think it’s a shame. That we have two different laws that are supposed to protect small firms, and we’re still not doing enough for them.
TY MONTAGUE
One of the other topics that you touch on is the topic of conflict of interest. One of the things that I just have a hard time getting over is government employees leaving the FDA and going to work for the corporations that they’re trying to regulate.
And you give a, a very poignant example of a person who wrote a regulation just so that they could jump out and get a job consulting on that same regulation to help corporations figure out how to win their way through it. Is that common?
RICHARD WILLIAMS
I, it, it’s hard for me to know. I don’t have data on how common it is. I certainly saw it often enough. The law allows it.
What they do say is it has to be a number of years before you can come back and lobby the FDA for that industry. But you can go out and work for ’em. You can tell ’em how to comply and you can make money. I could be wrong about this, but I think a lot of people feel, you know what? I work in a quote, unquote, low-wage government job. I should be allowed to go out and make money. Like everybody else.I sort of serve my country in this job, and I have some sympathy for that. but again, like you say, it seems to be something wrong. If you can be a part of writing a rule and then, you know, write it in such a way that you can go out and profit from it.
TY MONTAGUE
Yeah. I mean, this is a really hard question. So I don’t expect an answer honestly, but how do you solve that problem?
RICHARD WILLIAMS
It’s just a great question. I, I, I wish I, I did know, but I, I, I’m pretty sure it’s not the worst problem FDA has
TY MONTAGUE
What’s the worst problem?
RICHARD WILLIAMS
The fact that they’re not solving any problems. The fact that decades and decades go by, their, their budget keeps going up. They get more people. People believe in them. They believe that they’re keeping us safe. And that it just keeps going like that. And so when the fact that you can go to Congress every single year and say, we’ve got to do something one outta six, people are in this country getting sick from food poisoning every year for 30, 40 years.
You’re saying the same thing Congress goes, oh my God, that’s terrible. We gotta give you more money. well, no, say sooner or later you gotta start saying, okay, you gotta do something different.
TY MONTAGUE
So just to be fair, are there any big wins that you would point to times that the FDA has gotten it really right?
RICHARD WILLIAMS
Oh, a a absolutely. And, and first of all, let me say, lot of it is the system, it’s not the people. There are a lot of great people that work for FDA. They’re very smart. They’re very dedicated. They believe in it, but unfortunately they they’ve run out of ideas. But one thing I think, where we got it right, was trans fatty acids.
This was a case where initially we were just going to ask firms if they wanted to voluntarily label it. Trans fatty acids are worse for you than, than saturated fats. So that was the bottom line. We really needed to do something. So we kind of went round and round. There’s long story about it, but we ended up with mandatory labeling and companies sort of got the message and they began pulling trans fatty acid because they’re added to most foods.
So if they’re added, that means you can easily take them. As opposed to saturated fat, which is just a part of the food. So they started take them out. And then eventually, I think trans fatty acids adding them, has become illegal. I, I think that’s probably one of the best things that we did.
TY MONTAGUE
Is there anything that I haven’t asked you about that you think people should know about the FDA.
RICHARD WILLIAMS
I, I do think right now, with the commissioner saying our food safety system is broken, with what has been happening with infant formula, which FDA incidentally played a huge role in why that happened. for 40 years, they’ve been telling firms who want to come in, start making infant formula they said, no, they’ve kept it at six firms.
TY MONTAGUE
No kidding. So they created essentially the monopoly that
RICHARD WILLIAMS
Exactly. Exactly. So when you had one manufacturer drop out, of course, there’s a huge problem. So with, with these problems coming up, I think the curtain has fallen down and we see what’s going on behind the curtain. It couldn’t have a better time to start thinking about doing things a new way and, and actually trying to solve problems and just appear to be doing something.
So I’m hoping now is the time.
TY MONTAGUE
That’s a nice note to wrap up on. So let’s just say that you’re the FDA commissioner for a day or, or a month or a year for whatever period of time is necessary to make real change. What would you do to, help the FDA achieve its stated mission, which as it says on the website, it says “As in the past, the FDA strives above all else to safeguard the health and wellbeing of the American people,” what’s the one thing you would change about the FDA to help them that goal?
RICHARD WILLIAMS
I would, I would retask them. I would say, look, we’re not gonna pass all these regulations. Let’s start looking at these new technologies. If we think they’re not completely safe, that they need some sort of adjustment, let’s focus on those.
Let’s promote them. I I’ll just give you quickly a list of things. Again. Precision fermentation, genetic engineering. 3d printers are coming along. Consumer nutrition devices. They’re gonna have to go through the medical device, basically preapproval thing. We’ve gotta get those through faster. Those are gonna help consumers eat better.
Nano packaging, where we have smart packaging that’ll alert consumers when their food is becoming spoiled. All of these things, I would say, let’s start looking there. That’s the future. Those are the solutions. Let’s start looking at real solutions and stop trying to pass these regulations.
They, they were fine a hundred years ago. They’re not fine now. So I would, retask them.
TY MONTAGUE
Love that. Okay, Richard, on this show, we have a tool that we call the B.S. Scale that we use to measure the gap between word and deed. And our scale goes from zero to 100. Zero being the best. Zero BS and a hundred being the worst total BS. So what score would you give the FDA?
RICHARD WILLIAMS
Well, mostly I’m qualified to talk about the foods part of FDA. So I’ll just focus on them, on that scale because, you know, they say that they’re protecting consumers. They say all these things and they’re not doing it. I would give them about a 75. So it’s 75% of, of what they do in foods is bullshit.
TY MONTAGUE
Okay. Room for improvement. All right, Richard, thank you so much for being here today. This was a great conversation and I also wanna thank you for writing this book and doing the work that you’re doing post your time at the FDA. It’s incredibly important and we thank you for it.
RICHARD WILLIAMS
Thank you. Well thank you again for having me on. It’s been great.
TY MONTAGUE (VO)
Well that’s interesting, if not totally disconcerting. Earlier in the episode, Lauren Etter said that a significant issue with the FDA was it being under-resourced. Former FDA employee, Richard Williams, on the other hand, says that despite their consistent budget increases they still don’t accomplish anything.
If there was a harbinger of bullshit before there now seems to be a flashing neon arrow. But, to be fair, there is the biggest inter-agency left to explore.
Buckle up, folks, cause next we’re looking into the FDA’s Center of Drug Evaluation and Research. Right after this.
TY MONTAGUE
Okay folks. It is my great pleasure to introduce Dr. Gail van Norman, to the show. Gail Van Norman, welcome to Calling Bullshit.
GAIL VAN NORMAN
Well, thank you, Ty. It’s a pleasure to be here.
TY MONTAGUE (VO)
Gail Van Norman is a clinician and professor at the University of Washington. She writes and teaches about the medical research process – everything from the FDA to commercialization to how animal testing works.
TY MONTAGUE
So the reason that we are doing this episode on calling BS is that the FDA has come up. A couple of times on this show. And our research left me with the impression that the FDA sometimes gets things very right and sometimes it gets things very wrong. would you agree with that?
GAIL VAN NORMAN
I would, I think the, the FDA operates under constraints that are imperfect and it even the best of organizations tasked with such a complex mission are gonna have misses and hits. Overall the FDA has had a number of spectacular hits and pretty notable failures.
TY MONTAGUE
Yeah. So let, let’s get into some of those, cuz I’d, I’d love to have you start out providing, you know, an example or two of times when the FDA has truly lived up to their mission and really gotten it right?
GAIL VAN NORMAN
Okay, well, so let’s start with thalidomide because it’s a sort of a classic historical example of what can go right and wrong with medical research, as well as what happens at the FDA.
TY MONTAGUE (VO)
Thalidomide was an anti-nausea drug produced in Germany in the 1950s and it was considered one of the safest consumer drugs to ever hit the market.
GAIL VAN NORMAN
Because it was so safe, practitioners really picked out, its use in pregnancy because nausea during pregnancy is not only a misery to women. It can be, dangerous to the health of the mother and the fetus in utero. And so, having something that controls nausea and vomiting is very important. The drug had been tested in animals prior to human tests, it had been tested. And I, I believe the number is something like 50 or a hundred different species of animals, including rats and mice and dogs and cats. And. Armadillos and ferrets and rabbits. It was considered such a safe drug that it was not required that you have a prescription to use it. the drug manufacturer gave it away free to its factory workers, pregnant factory workers to use during pregnancy.
TY MONTAGUE
Wow.
TY MONTAGUE (VO)
Wanting to expand the market into the US, the manufacturer submitted the drug for review with the FDA. The committee reviewing Thalidomide was headed by Frances Oldham Kelsey, who just so happened to be the first woman to hold the position.
GAIL VAN NORMAN
She later joked that they gave her what they thought would be the easiest one they could possibly give.
And whether they did that because she was a woman or she was new, we’ll just leave to speculation. Anyway, she read the data and something about it didn’t ring true with her. She didn’t like it. And she said, I’m not gonna approve this. I’m gonna stop you. And I wanna see a few more studies. And, just a few months later on Christmas day in 1956, the first baby was born in Germany without ears – a little baby girl, and that was followed by over 10,000 cases of severely deformed infants that were born in probably 20 to 30,000 cases of in utero deaths. And the us saw exactly 17 cases of thalidomide deformities. Presumably in the children of mothers who brought the drug in from out of country, we were saved that plague, because we never approved the drug in the United States for that use.
TY MONTAGUE
I mean that actually I had a galvanic response to that story. Like it makes the hair on the back of my neck, stand up to make me think how close we came to a total unmitigated disaster, you know?
GAIL VAN NORMAN
Yeah, it, it, it really is true.
TY MONTAGUE
And let, let’s talk about COVID also because that’s fresh in everyone’s mind and that’s clearly a case where, you know, I mean, it, it seemingly the normal process takes forever and it just, it feels like COVID vaccines just magically appeared. How did that happen?
GAIL VAN NORMAN
It was a miracle.
TY MONTAGUE
Incredible right?
GAIL VAN NORMAN
I’m, I’m, I’m thinking to myself two and a half years ago when COVID hit. And I have in my distant medical background, I have a background in immunology. And, I remember people coming and asking me, well, how long will it take for us to have a vaccine? And the average time to get a vaccine created for a new disease is 15 years. So it’s, it was like, so right away, what was happening was the, the, government was saying, we’ll have a vaccine for you in a year. And I was going not on your life. You won’t.
TY MONTAGUE
Right. No chance.
GAIL VAN NORMAN
No chance.
TY MONTAGUE (VO)
But of course, there WAS a chance. Due to several important factors all occurring simultaneously. First, the U.S. government created a public-private partnership offering $10 billion to pharmaceutical companies to start immediately making and testing vaccines.
Second, MRNA vaccines had become available. This new technology created a way for the body to show a facsimile of the virus to itself, making testing on humans easier. And, finally, the 21st century cures act accelerated medical product development via new statistical models.
GAIL VAN NORMAN
All of those things came together. It’s just amazing.
TY MONTAGUE
Yeah, it is amazing.
TY MONTAGUE
So thalidomide and COVID vaccines. Yay. FDA, truly. And, and they do so many more things right as well. Those are just a couple of examples, but let’s, let’s pivot to where they get it wrong. For, for me, because we did the research we did, Purdue pharma comes to mind and the Oxycontin crisis.
So, the thing that’s that shocked me about the Purdue situation and I did not, I did not understand this, Prior to doing this research, but there are people at the FDA who have to write the language that goes on warning labels on drugs.
And there are different kinds of warning labels and The big thing in, in Purdue’s world, Oxycontin was, addictiveness. Any opioid heretofore had been deemed to be addictive and had to carry a label that said it was addictive and, and would be prescribed in that same way, i.e. very sparingly, and Purdue figured out a way to get the warning label written in such a way as to avoid any language of addiction, so that more doctors would be inclined to write prescriptions for it.
And my understanding of this story is that the person who wrote the language at Purdue actually holed up in a hotel room with the executives from Purdue and they all crafted that language together. And so this gets to a larger issue obviously, which is sort of the revolving door or the, I would say fraught relationship between giant for-profit industries and low paid government workers.
And further to sort of add insult to injury. Once the language had been crafted, once Oxycontin was approved and being sold across the world, that person left the FDA and took a job at Purdue pharma.
Kind of closing the circle as it were. And you know, that story really disturbed me. it outraged me honestly. And I would love to hear your take on that and how widespread a problem like that really is.
GAIL VAN NORMAN
I think what you’re getting at is a real problem in that there is variously porous interface between the kinds of researchers who work with and within the FDA and those that work in commercial industry. And so people do switch teams.
TY MONTAGUE
And that’s perfectly legal right now, is it not?
GAIL VAN NORMAN
Oh sure. I mean the current commissioner of the FDA, Robert Callif, was an executive for- I can’t remember the name of the research company that’s a subsidiary of alphabet. Anyway, he made 2.7 million a year in salary and he used that- and I’m not criticizing him I don’t make it make it sound like it was nefarious- that was actually a selling point for his appointment as the commissioner to the FDA, because he knew the ins and outs of commercial research companies. So you can make an argument that it’s helpful and by the way, his salary there right now is now $300,000, which is considerably less.
And he put in a lot of agreements to say he would not participate in owning selling, talking to, you know he self-restricted to say, I’m, I’m gonna make sure you know that who I work for.
TY MONTAGUE
Right.
GAIL VAN NORMAN
You know who my boss is, but other people have not been quite so clear about it. And so you have somebody who works on an FDA committee and they have a drug presented to them and the people who are presenting the drug whisper in their ear. Wow. We really think your help has been really valuable. And we’d like you to think about coming on as a researcher or a head of marketing in our company, and they offer you a two and a half million dollar raise. It can be a little hard to turn that
TY MONTAGUE
down, right? Yeah.
GAIL VAN NORMAN
And so there are connections which between the FDA and the commercial world, which if properly aligned are helpful, because it can give the FDA insights as to what the company’s doing, but if they’re not properly aligned, can lead to conflicts of interest and to the detriment of the safety and health of the American public.
TY MONTAGUE
So the FDA is a government organization with a mission to protect us all from the effects intended or not of private for-profit interests. So, so let’s talk a little bit about money. How does, how does the FDA receive its funding?
GAIL VAN NORMAN
Well, the FDA used to be back in the 1920s. It was all government funded. It just came out of the general treasury fund for the FDA. Now about 45% of the FDA’s funds come from something called user fees and application fees that are paid by the drug companies to get their drugs reviewed.
When that was first proposed, there was a lot of concern that this would create a conflict of interest for the FDA, because a lot of their funding would come from the very people they’re trying to.
TY MONTAGUE
Regulate. Yeah.
GAIL VAN NORMAN
But it became so popular because, those user fees were enacted because the FDA was woefully short staffed. Congress enacted this as a way specifically for the FDA to hire the people that needed to do the work. And it led to a big reduction in throughput times, and it accomplished exactly what it’s supposed to do, so it’s still popular to this day.
TY MONTAGUE
And how much do you think the FDA is affected by lobbying either by, big drug companies lobbying on the hill to change laws and regulations or patient advocacy groups lobbying to influence, the way the organization is, is funded or regulated itself.
GAIL VAN NORMAN
Well, both affect the FDA. I mean, it’s not immune to them. The FDA ACTS is funded, as I mentioned, 55% from congressional funding and Congress is affected very heavily by patient advocacy. You get patient advocacy groups, getting Congress to write both good and cockamamie laws all the time, with regard to health. And so the FDA is not immune from those effects.
The other thing I would say that there’s no, it’s hard to say that there’s a direct effect.
Like, you know, GlaxoSmith Klein plays their 2 million drug fee and that makes the FDA approve the drug. That doesn’t happen.
But the other kind of effect, is hard to regulate against the individual who sits in an influential part of a committee who then is favorably impressed by a drug company about their drug in various ways and advocates for it. Those do happen and individuals that sit on these committees can make big differences, in what the FDA decides to do.
TY MONTAGUE
One of the things, that is just true is that our healthcare system is run largely as a for-profit enterprise. And is that part of the problem here?
GAIL VAN NORMAN
Oh, sure.
TY MONTAGUE
Or is that the whole problem here?
GAIL VAN NORMAN
That might even be the whole problem for all we know. I mean, I suppose there are good aspects to commercialization because it does, in an ideal world, promote competition and innovation. But it also promotes manipulation and profit taking and neither of those serve patients at all, they serve the companies that make the drugs
TY MONTAGUE
And their shareholders
GAIL VAN NORMAN
Talking about,well, that’s right.
I mean, we’re not just talking about the, the company making a profit we’re talking about unimaginable amounts of money. We’re talking about a drug generating a hundred billion in profit, a hundred billion dollars. So this is money that will buy anybody’s soul, right?
maybe even mine, I don’t think so, but you never know
TY MONTAGUE
Well, and no, like
GAIL VAN NORMAN
You know,
TY MONTAGUE
It’s an interesting question to ask yourself, right. Is like, what’s my number? in the current system, the consequences for breaking the law often take the form of fines, but with profits of that size, you know, would it be fair to say the fines are just a cost of doing business?
GAIL VAN NORMAN
Oh, absolutely. it it’s, it’s amazing. And what you see in the media, what the American public sees is the Sacklers paid, what was it? 13 billion. Billion dollars well weighed against what did they make? Even if it’s
TY MONTAGUE
In the
GAIL VAN NORMAN
A hundred, over a hundred billion, it’s less than 13% of their whole profit margin.
And remember it’s not just the profit, it’s not just the actual dollars that go in the bank. It’s how much their stock is worth So if you’re making a hundred billion dollars in profits, your stock becomes worth a, a huge amount more. And these companies are run by individuals who have heavy stock interests who may have individual conflicts of interest with doing the right thing.
TY MONTAGUE
Right.
GAIL VAN NORMAN
And more and more we’re talking about dollar amounts that seem insurmountable in their ability to bribe and attract people into behaviors that we would hope we wouldn’t see in this industry.
TY MONTAGUE
Right. And, and, you know, just to contrast that to make this really clear, how much money would a typical FDA employee take home, if there is such a thing. And how much can we contrast that with an average pharma executive? Even just roughly?
GAIL VAN NORMAN
Well, I, I, the pharma executives take home millions of dollars. The average FDA salary is $110,000 a year. And their top executive makes $300,000 a year. So it’s considerably less than anything. The commercial environment can offer.
TY MONTAGUE
Yeah. And it just seems like there’s a massive incentive for these big companies to try to figure out how to game the system in one way or another.
GAIL VAN NORMAN
Well, I, I think it’s not just, there’s this incentive. There’s virtually no disincentive to do it because if you are that rich, you can just pay the fine and move on
TY MONTAGUE
It doesn’t even matter. Yeah.
GAIL VAN NORMAN
Doesn’t matter, you actually factor that into the cost of developing the drug, the cost of putting the drug out there.
TY MONTAGUE
Yeah, well, that’s terrifying. So, Gail, another way to look at our show is fundamentally it’s about trust. And it’s just incredibly important that we trust institutions like the FDA- literally lives are on the line.
And honestly, I can understand someone who has lost trust in politicians today or in big pharma, or even in the FDA. You know, this is the same institution that approved Oxy. So what are some things that you think the FDA should do to try to rebuild trust with people?
GAIL VAN NORMAN
I think, first of all, we need to start with Congress and say, who do we have in Congress that is sitting on the committees that give the FDA its marching orders?frankly, I. I can’t tell you who those people are. I should be able to, but I can’t right now. And do I trust them, particularly when I well know that many of our congressional representatives are for lack of a better word ignorant of science, and how it works and don’t care to learn it and pander to, sort of the conspiracy theorists who want to think that we’re all out to get ’em.
So I think, we need to look at that and ask, should there be special qualifications for people who determine how this agency works?
GAIL VAN NORMAN
I think overall the agency does a remarkable job given the mission that it has and the number of opportunities for failure that it has, how relatively few, it, it really has experienced.
I think we need to look at how we can reduce conflicts of interest that we’ve talked about within the agency, so that we don’t have people who are pretending to serve the FDA, but are really serving a commercial interest or serving themselves so that they can position themselves for a, for a well paying job with the commercial companies I think that would be helpful. And I think we need to have real penalties for pharmaceutical companies that openly commit criminal acts. This is in the criminal code now
TY MONTAGUE
Yeah.
GAIL VAN NORMAN
Purdue did things that were criminal.
TY MONTAGUE
Yeah,
GAIL VAN NORMAN
And, that perhaps money is not the price. Those people should be paying, that
TY MONTAGUE
Like jail time. Right?
GAIL VAN NORMAN
There should be real prison time assigned when those sorts of things happen. Because a CEO who knows that their signature on a piece of paper might put ’em in jail one day, may think twice before signing it.
TY MONTAGUE
Absolutely. And you know, it’s not.
GAIL VAN NORMAN
Better oversight.
TY MONTAGUE
Yeah. Okay. All right, Gail. this is really my last question. On this show, we have a tool called the BS scale and the BS scale goes from zero to 100 zero. Being the best score, meaning zero BS, 100 being the worst, total BS. So on that scale, what score would you give the FDA in achieving its stated mission?
GAIL VAN NORMAN
I would give it a really good score. I think that to do it’s the complicated job it does to do it with the high degree of success that it’s had in protecting the American public for nearly 200 years now. I that they deserve a score of a 25 and that they’ve done really, really well.
TY MONTAGUE
Gail, I want to thank you for being with us today. Thanks for calling bullshit.
GAIL VAN NORMAN
Well, thank you for having me. It’s been fun. It really has been.
TY MONTAGUE (VO)
Folks it’s time to make the call. The FDA is a complex institution with huge responsibilities. We couldn’t possibly cover the entirety of what they do in a single episode – but with the help of our three experts we’re still able to get enough insight to answer the question- does the FDA strive above all else to safeguard the health of the American people?
Although there are some notable successes – we’re calling bullshit on the US Food & Drug Administration.
But, as always, we’re not here just to curse the darkness. When we come back we’ll speak with an expert in FDA conflicts of interest to see if we can light some candles in the halls of this bureaucratic behemoth.
GENEVIEVE KANTER
My name is Genevieve Kanter. I am an assistant professor at the Perelman school of medicine at the university of Pennsylvania. I’m trained as an economist and I study regulation of biomedical technologies, the FDA and conflicts of interest.
TY MONTAGUE
Thank you for being here and welcome to Calling Bullshit.
GENEVIEVE KANTER
Thanks. Happy to be here.
TY MONTAGUE
What we’re here to do is talk about, ideas for helping the FDA better live their purpose. And before we get into those ideas, I don’t do this in every show, but, but I wanted to actually say something to our listeners because I’ve been feeling some of this stuff myself, as I prepped for this episode.
It’s really easy. and, and I would say even understandable when faced with a problem as complex as the FDA to basically just shrug and give up. And it’s, it’s easy to just declare that the problem is impossible and to kind of move on. And I want to ask our listeners to suspend their disbelief for this section, because we really do want to explore actions that the FDA or the executive branch to whom the FDA reports could actually enact to help the FDA build better trust with people and really deliver on the promise of keeping all of us safer. It’s such an important purpose. And I really believe we need to take an optimistic point of view here, because giving up on it is unthinkable. So, so I just, sorry, I had to get that off my chest initially. so let’s get into some ideas,
Genevieve. I’m gonna ask you to go first. In two minutes, can you tell us the one thing that you would do to change the FDA?
GENEVIEVE KANTER
So if I were emperor of the United States, the one thing I would do is to replace our current approval process with a system where the firms are given conditional approval of their products, and they have to seek renewal of approval every say, 10 years. So in the current system, a firm applies for approval of a product and receives that approval until basically the end of time or the sun burns itself out or something.
So with this 10 year sort of renewable approval, you could incentivize the monitoring of how well drugs are working, because that will be required to get your renewal for the approval you incentivized the monitoring of how safe drugs are. Because again, that’s part of the renewal process that will allow us the government to pull drugs off the market that turn out to be not effective or that turn out to be unsafe because sometimes you don’t see a lot of safety events in the small clinical trial populations, and you only see them in the broader population.
TY MONTAGUE
I love that idea. That is an incredibly smart idea. Not surprisingly, you are the expert so well done. I, I think that’s a fantastic idea. We, we will, return to that. I’m sure throughout the conversation. So here’s my idea. The FDA is much more important to all of us than is currently reflected in the salaries of the people who work there.
Their job is literally to, to save lives, to protect us from harm. but there’s a pretty massive salary gap between the average FDA worker and the average pharma exec or food company exec, which has resulted in this revolving door where regulators from the FDA move to higher paying jobs at food and drug companies.
And I think that the knowledge that that reward is there waiting for them, if they play ball while they’re at the FDA, has the effect of creating huge conflicts of interest. And so my idea is to change the FDA through compensation reform. Pay salaries competitive with the private sector. Because right now the FDA is kind of a drab government bureaucracy, and it attracts people who are up for working in that kind of job.
Closing the salary gap would begin to level the playing field. When you know, you pay people more money, you attract better people, and their job is so incredibly important that that would be better for all of us. So salary reform is the idea I want to put on the table, but let’s let’s we can, we can get back to that.
I want to, I want to talk more about your idea because I think it’s so smart. Because when things and, and a lot goes right at the FDA, right? But, but when things go wrong, it’s not often on day one. You don’t know that a problem is a problem right out of the gate. And yet, once something is approved, it’s been released into the wild and you almost can’t, get it back, in the current system.
And so that, I think that would, you know, really increase, people’s safety. What barriers do you think we would encounter if we decided to try to actually enact that today? Who would have an issue with that idea?
GENEVIEVE KANTER
Probably the same parties that have blocked a lot of reforms in this space. the pharmaceutical companies. I suspect that some of the arguments that would be presented might be that it would be even more costly and time consuming for firms than the existing approval process is.
Uh, it might delay, access to some products. but overall, broadly speaking, it’s, not politically feasible because, the drug companies would, intensely oppose this kind of, conditional approval.
TY MONTAGUE
And when they oppose that kind of thing, how does, what form does that opposition take?
GENEVIEVE KANTER
So, every five years, there is legislation related to, authorizing, the budget for the FDA. Um, in fact, there’s actually currently, the reauthorization happening this year and we expect to see it passed um, at end of the summer, actually. And so usually it’s through, you know, lobbying, legislators, as to the features that might go into this reauthorization package.
So if it, for example, if it were to be introduced in one of these five year reauthorization bills, pharma as well as individual pharmaceutical lobbyists would oppose the inclusion of, of such a conditionality.
TY MONTAGUE
And the lobbyists are there to threaten by removing financial support from Congress,
GENEVIEVE KANTER
That’s right through, you know, campaign, uh, contributions.
TY MONTAGUE
Hmm. Yeah, that sort of leads us to a discussion around conflict of interest in general. So, so I want to go there, but before we go there, more deeply, what, what do you think of the idea that I put on, on the table? This idea of like leveling the playing field from a salary standpoint, does that make any sense?
GENEVIEVE KANTER
I like it and I think you’ve tackled head on one of the issues with the approval process at the FDA, which is they do lose a lot of very good people, because the pharmaceutical companies are able to, you know, entice, government workers who have a lot of experience, and knowledge, away.
I do see, some constraints while we’re on the topic of, you know, pluses and minuses. one might be that, these are civil servants. And so what you describe is just a generic problem among the civil service. So are there some rules related to parity relating, you know, GS scales and so on that you would have to consider, a
TY MONTAGUE
I see just the way government workers are compensated, has to be essentially universal. They’re
GENEVIEVE KANTER
Or standardized? Yeah. In some way. A, a second, issue is just where that money would come from. we, because a central source of conflict, actually, even with funding, the FDA is, user fees. So basically requiring pharmaceutical companies to pony up, you know, hundreds of thousands of dollars to finance the review process.
Now it’s not earmarked so that if Pfizer submits a, you know, drug for review that, you know, people will, necessarily favor the approval of that drug, but financially the FDA and its operations are, funded in large part by pharmaceutical companies through these user fees.
TY MONTAGUE
I, I would consider that a tax in a way of, getting your product to market. And, I, I think that, that makes sense. I mean, I do realize it gives them a voice in the world of, of money and politics. and so raising those fees probably would be unpopular with them. I want to continue to talk about different conflicts of interest. you wrote a chapter in a book called conflicts of interest in FDA advisory committees. that was eye-opening for me. Can you first just explain what an advisory committee is and, and how that actually works?
GENEVIEVE KANTER
When, a drug comes up for approval, the FDA, you know, has the final say, but oftentimes it doesn’t have the internal expertise or perhaps even the person hours to commit to doing a full review, of a particular drug or, you know, the evidence is complicated and it needs external advice. So frequently it convenes these advisory committees to review the application for particular drug.
The people on these advisory committees are not formally employees, or full-time employees of the FDA, they are external experts. They’re sitting at universities, you know, research institutes think tanks, and they are physicians, researchers, some statisticians.
But one of the important things about this is, if you work for the government, there are very clear ethics rules regarding your financial ties to industry. If you are working at a university and then you get called on to be on these advisory committees, You know, you, these people sitting at universities have relationships with drug companies.
They, are consultants for them. They have the research funded by them. And so one of the things I looked at, was whether the financial ties of these external experts, who are called upon to advise whether a drug should be approved or not, whether the financial ties that these experts had to drug companies was associated with whether they voted for approval of the drug or not, and how open they were to approval of the drug.
TY MONTAGUE
Right. And it sounded to me like you had a specific hypothesis going into the work, which actually you even, you were surprised by the results. Is that right?
GENEVIEVE KANTER
Yeah. So, I mean, conventional wisdom, certainly in the ethics literature, on conflicts of interest, which is if you have, you know, one tie to industry, you know, that’s not good. but if you have multiple ties, that’s even more, not good. That’s like really, really bad. Uh yes, exactly. And, and so we found two things.
One was, it turned out that, when we compared how these experts voted and we compared people who had one financial tie, to those who had no financial ties, uh, we actually found that, people with a single financial tie to a company were more likely to vote in favor of the product, uh, sponsored by that company, than people who had no financial ties.
So there did just seem to be some bias, but it was only if you had a single financial tie to a company. In contrast, people who had a lot of financial ties. So people who had ties to Merck Pfizer, lots of companies did not appear to vote any differently on average than people who had no financial ties.
So people with a lot of ties, did not appear to be biased in how they voted. So we talk a little bit about why that might be the case. One, you know, hypothesis that makes sense to me is that, you know, a lot of times when people have a lot of financial ties, it’s because they’re really, really good at what they do.
And so a lot of companies want a piece of their brain. It’s, it’s not, so they’re not hiring these people so they could be hired guns to say what the company wants them to say, they’re hiring these people because they’re just really good at advising them.
TY MONTAGUE
Some of it is just, they literally want great advice from smart people.
GENEVIEVE KANTER
But we did find some bias. I mean, I think the other thing that came out in the paper was that, you know, the type of conflict matters. So the hypothesis that I presented earlier, one tie is bad, many ties, worse, is sort of. The simplistic rule of thumb, we had that doesn’t acknowledge the fact that different kinds of ties matter for influence. And so the other thing we found was that it did not seem for example, that experts who had ties through research funding. So their research was funded by the drug company, were biased, but, the kind of financial ties that really mattered were, either you were, you had an ownership stake in the company, you had, some kind of stock in the company, which makes sense and, also a very strong effect came from whether you were on an advisory board for the company. Lots of times you may be on the board and you have a fiduciary responsibility, for the company to act in, uh,
TY MONTAGUE
In the country in the company’s best interest, right? Yeah. Yeah. That’s a clear conflict like that. That seems like that should be illegal. I mean, a lot of this should be illegal to be honest.
GENEVIEVE KANTER
You would think it would. and if you read the rules, people who do have that kind of financial interest, um, should not be participating in the advisory committees, but there is also, a process that allows the FDA to make exceptions. And many times they make exceptions.
And so you have people with these kinds of financial ties on these advisory committees, but things that don’t matter, consulting, research, you know, other kinds of ties don’t matter. ,So I guess the paper was really advocating for more subtle policy related to conflicts of interest.
TY MONTAGUE
More disclosure, right? I mean, it seems like that should be fair. If the FDA is tasked with, as it says on the website, above all else, safeguarding the health and wellbeing of the American people, if that truly is above all else, then you know, you should have to disclose all of your financial ties and it should be acknowledged that there are financial ties that are okay.
In other words, if you’re just being paid as an advisor for a company that’s you being paid for your professional expertise, and that’s fine. If you own a piece or you are on the, on the board of one of the companies that’s in question that would disqualify you.
And I don’t understand why we can’t enact rules like that. What would prevent us from closing those loopholes?
GENEVIEVE KANTER
That’s a great question. The two arguments that I’ve seen presented are that, we would no longer be able to find people qualified enough to be on our advisory committees. If we just outright banned the participation of people with financial ties.
TY MONTAGUE
Because all the, all the really smart people are already on the take, essentially like.
GENEVIEVE KANTER
Or, you know, whereas my study indicated, you know, a lot of people want, want a piece of their brain. So, it would difficult and, we can kind of see this because, in the two thousands, the FDA in fact, capped the exceptions that could be issued for people who had these kind of financial ties.
Um so, prior to this, the FDA issued just exceptions willy-nilly and just basically said a lot of the apparently disqualifying financial interests didn’t matter. In the two thousands with one of reauthorizations, the percentage of people who had these exceptions and what you saw was of course, a decline in people who had these exceptions and these financial ties, but what you also saw was, more positions on the advisory committees being vacant, a longer time.
it would take to convene these advisory committees because they would need to spend more time to find people who were not conflicted.
TY MONTAGUE
I wanna, I wanna delve a little bit more into this idea of trust because ultimately the FDA’s job is to make us safe and, without guiding your answer at all, what needs to change so that we can trust, the companies that are making our food and the companies that are making our drugs? What, what about our system has to change to create more trust?
GENEVIEVE KANTER
I have to say I’m a little conflicted about this. I mean the mission of the FDA is pretty clear, you know, to ensure among other things, to ensure the safety and efficacy of the drugs that are marketed in the us, the mission, the drug companies is not that, it is to make money for their shareholders.
And so, you know, I do wonder whether it’s realistic, to expect organizations whose objective is profit making. To not do what they can do to make profits. but I like the way you framed it because you framed it as a system, you know, not just the FDA, but, I think the onus is on legislators policy makers to create rules that put guardrails on drug companies that still, but that still incentivize them to do the right thing.
I think as, as you, you might have framed it. so things that minimize gaming, how clinical trials are run and analyzed things that incentivize the collection of data on effectiveness and safety. And so to tie the things that we want, which is quality information on safety and effectiveness, with things that drug companies want, which is, you know, access to the market.
TY MONTAGUE
Genevieve, is there anything else, any, are there other topics or anything else that you think our, our listeners should know about, the FDA and things that, that either could or should change?
GENEVIEVE KANTER
I do think, the problems we have with the FDA are structural, you know, there is that structural tension there about how can we get, how can the agency have independence be based on the science, but, you know, still have a commissioner that is, you know, serving in the pleasure of the president. the other structural tension. And we see this, you know, in our discussion as well is, you know, you can have an agency that approves drugs almost too fast.
So they prove drugs that don’t work, or you have safety issues, but you’ve increased access to the drug, but that’s opposed to, well that, you know, the alternative is to have, an agency that approves things too slowly, which is there are drugs that it’s preventing from being out on the market that people need to get access to
TY MONTAGUE
Right.
GENEVIEVE KANTER
We do need to have information systems that adapt and approval system that is adaptive in the same way that, you know, all other systems in the world that we live in, you know, adapt to new information. and so hopefully that proposal and yours as well, sort of adapts to, you know, what we know, and we can make better decisions that way.
TY MONTAGUE
Okay. So last question on this podcast, we have a tool that we call the BS index and the BS index measures the gap between word indeed. And it goes from zero to a hundred. Zero is the best score. So zero BS, a hundred is the worst score, total BS.
And so the FDA today says that it strives above all else to safeguard the health and wellbeing of the American people. What score would you give the FDA?
GENEVIEVE KANTER
So I would say that my determinate of the BS score is based on two main things that I think caused me to be worried about the FDA. One is the degree of industry influence that leads it to diverge from its mission, as well as, the degree to which it’s vulnerable to political influence, you know, from the executive branch.
Overall, I, I think the structure of the organization, gives us generally reasonably high quality decisions. I. Give it a 45.
TY MONTAGUE
Okay. that was great. Thank you so much for being here today, Jenny. I really appreciate it.
GENEVIEVE KANTER
Thank you for inviting me and having us, Think together, about this issue, it was, I really, really had a great time. Thank you.
TY MONTAGUE (VO)
Alright folks – it’s time for the FDA to officially get their BS score. It somehow feels appropriate that I try to get a little scientific with this one so, if I were to average our guest scores the final score would be 48. However, that feels low to me, especially given Richard’s account of basically being told to deliver the results his bosses wanted or risk losing his job at the agency. That’s just plain wrong. So I’m going to give the FDA a 55. I hope that acknowledges some of the agency’s big wins over the years, but leaves PLENTY of room for some much needed improvement.
TY MONTAGUE (VO)
FDA Commissioner Robert Califf – if you ever want to come on the show to discuss anything we’ve touched on today, please know that you have an open invitation.
And if you’re starting a purpose-led business or thinking about beginning the journey of transformation to become one, here are three things you can take away from today’s episode:
TY MONTAGUE (VO)
And if this episode made it through your approval process subscribe to the Calling Bullshit podcast on the iHeart Radio app, Apple Podcasts, or wherever you listen to people speak into your ears. And friends, I’d like to ask for your help if you enjoy the calling Bullshit podcast please take a second to rate us on Apple podcasts or on your preferred platform.
Thanks to our guests Lauren Etter, Richard Williams, Gail van Norman, and GENEVIEVE KANTER. Learn more about them and get links to their work in our show notes.
And, many thanks to our production team. Hannah Beal, Amanda Ginsburg, DS Moss, Haley Paskalides, Parker Silzer, and Basil Soper.
Calling Bullshit was created by Co Collective and it’s hosted by me, Ty Montague. Thanks for listening.
Tagged as: fda.

Calling Bullsh!t October 19, 2022
Unilever’s purpose is “to make sustainable living commonplace.” The sheer size of this company makes that an especially difficult task. But it also means that when they do meet their […]
Don’t agree with our Bullshit Score?
Give us your take.